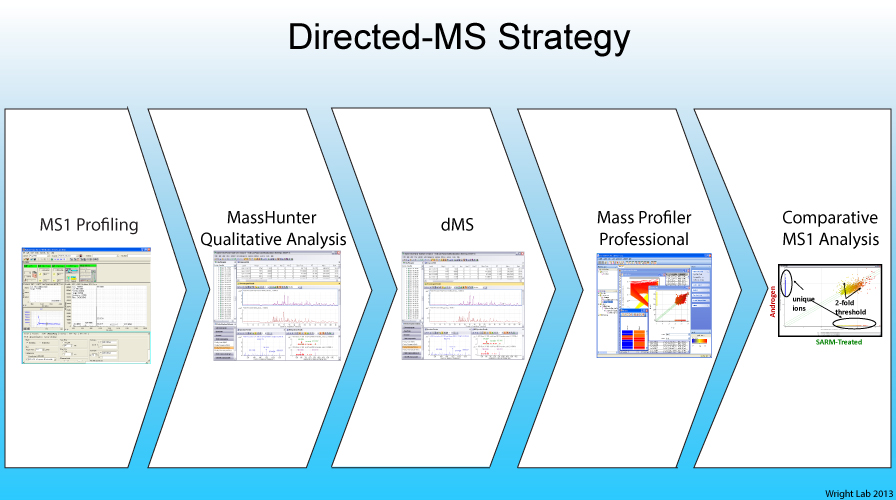
Our laboratory uses quantitative mass spectrometry as a platform to delineate signaling networks in normal and diseased biological systems. The goal of current mass spectrometry-based proteomic workflows is to sequence every gas-phase peptide ion present in a complex biological sample using data-dependent tandem mass spectrometry. This approach, which is commonly referred to as "shotgun proteomics", is the primary workflow for interrogating low- and high-complexity protein samples. However, recent advances in the software needed to acquire, control, and process MS spectra on high-resolution mass spectrometry instruments have catalyzed the birth and evolution of directed and targeted mass spectrometry (MS)-based proteomic strategies
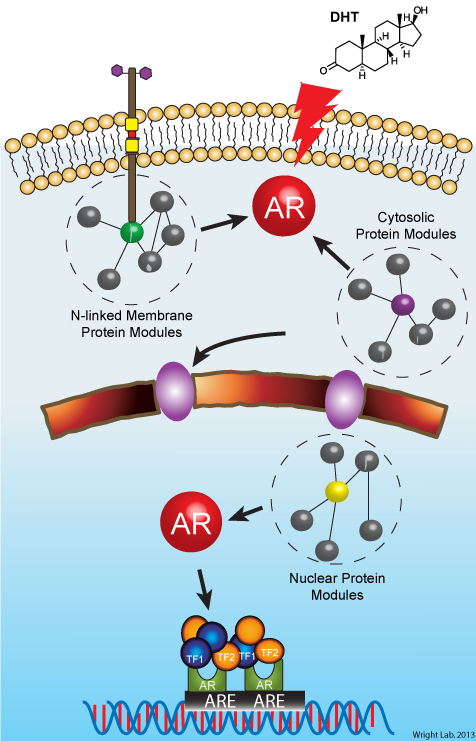
We are studying the molecular and cellular actions of androgen signaling pathways in normal and diseased organs/tissues. A major goal of the laboratory is to build quantitative models of androgen-mediated signal transduction that predict both the physiological and pathophysiological actions of androgens, at the molecular and cellular levels.
These quantitative models of androgen signaling will guide our understanding of how steroid hormones impact cellular responses in different organs and tissues, and also provide a roadmap for targeting or modulating the pathways that are under the control of androgenic hormones. We are initially focused on determining how androgen signaling pathways influence the tumorigenic and metastatic potential (as assessed based on cell proliferation, survival, adhesion and migration) of human prostate cancers.
The androgen receptor (AR), a member of the steroid hormone-receptor superfamily, is the primary receptor for androgenic ligands in normal and cancerous prostate epithelial cells. Years of biomedical research have led to the general understanding that aberrant AR function is the primary defect in both hormone-naïve, organ-confined prostate cancers and metastatic, castration-resistant prostate cancers. Given this link, my laboratory is actively building molecular maps of AR-signaling events related to the etiologies of both cancer types, and investigating the spatial-temporal consequences of AR activation and its pathological effects in cellular models of each. On-going projects include:
Careful characterization of these pathways will provide an integrative molecular understanding of how androgens control AR-signaling pathways at the molecular level. Our findings will allow us to understand how disparate signaling pathways interface with AR-signaling pathways, and provide new insights into the biochemical connectivity of androgen-AR signaling responses at the molecular and cellular levels. This information will bridge gaps in our current understanding of how aberrant AR function influences the tumorigenic and metastatic potential of human prostate cancer. We also expect these findings to open up new research avenues for dissecting and understanding the molecular outcomes of androgen signaling responses in other hormone-responsive organs and tissues in the human body.
Our group is also interested in identifying biomarkers that will distinguish indolent from significant disease in patients afflicted by low Gleason grade, organ-confined prostate cancer. To this end, we are utilizing directed and targeted MS workflows to quantify the relative expression of cell surface receptors in cancer samples and the surrounding normal tissue. This research is expected to identify a cohort of cell surface receptors that will predict the clinical outcome in patients with low-grade disease, and thus reduce the overtreatment of prostate cancer patients with low-risk, organ-confined prostate cancer.