Lab Research
Electrical signals generated in the cell membrane of nerve and muscle send messages to the cell interior via the opening of voltage-gated Ca2+ channels. The resulting Ca2+ influx forms a signal that can activate or inhibit numerous pathways including gene transcription, protein phosphorylation, and vesicle fusion. Where, when, and how much Ca2+ fluctuates in a cell can determine which pathways are activated. However, large increases in Ca2+ can cause cell death. Therefore, cells utilize multiple mechanisms to regulate Ca2+ influx through voltage-gated Ca2+ channels. Our lab studies these mechanisms and their roles in the heart, brain, retina and inner ear:
Neuronal and cardiac Ca2+ channels
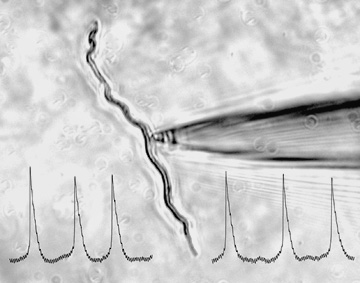 In the pacemaking cells of the heart, Cav1.3 Ca2+ channels are required for normal cardiac
rhythm. In the adult brain, Cav1.3 regulates repetitive firing of dopamine neurons and
Ca2+ signals that increase vulnerability of these neurons to degeneration in Parkinson’s
disease. Thus, factors that regulate Cav1.3 may control the balance between normal and
diseased states of the cardiovascular and nervous systems. We have found that that PDZ-
domain containing proteins, such as erbin (Calin-Jageman et al.) and densin
(Jenkins et al.), are versatile modulators of Cav1.3. We are studying the
molecular mechanisms underlying PDZ-protein regulation of Cav1.3 and their roles in regulating
spontaneous action potentials in cardiac myocytes and neurons.
In the pacemaking cells of the heart, Cav1.3 Ca2+ channels are required for normal cardiac
rhythm. In the adult brain, Cav1.3 regulates repetitive firing of dopamine neurons and
Ca2+ signals that increase vulnerability of these neurons to degeneration in Parkinson’s
disease. Thus, factors that regulate Cav1.3 may control the balance between normal and
diseased states of the cardiovascular and nervous systems. We have found that that PDZ-
domain containing proteins, such as erbin (Calin-Jageman et al.) and densin
(Jenkins et al.), are versatile modulators of Cav1.3. We are studying the
molecular mechanisms underlying PDZ-protein regulation of Cav1.3 and their roles in regulating
spontaneous action potentials in cardiac myocytes and neurons.
Auditory Ca2+ channels
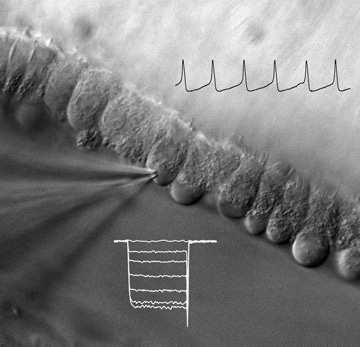 Hearing starts with the transmission of sound by inner hair cells (IHCs). In these cells, Cav1.3
Ca2+ channels mediate Ca2+ action potentials before the onset of hearing and Ca2+ signals that
trigger exocytosis of neurotransmitter from IHCs onto auditory nerve afferents. These functions
of Cav1.3 are crucial for the development and maintenance of hearing: mice lacking Cav1.3
are congenitally deaf, as are mice with upregulated Cav1.3 channels due to thyroid hormone
deficiency. Thus, factors that regulate these channels can profoundly impact this first synapse
in the auditory pathway. This project screens for novel modulators of Cav1.3 (Cui et
al.), which may fundamentally influence the function and localization of these channels in
IHCs.
Hearing starts with the transmission of sound by inner hair cells (IHCs). In these cells, Cav1.3
Ca2+ channels mediate Ca2+ action potentials before the onset of hearing and Ca2+ signals that
trigger exocytosis of neurotransmitter from IHCs onto auditory nerve afferents. These functions
of Cav1.3 are crucial for the development and maintenance of hearing: mice lacking Cav1.3
are congenitally deaf, as are mice with upregulated Cav1.3 channels due to thyroid hormone
deficiency. Thus, factors that regulate these channels can profoundly impact this first synapse
in the auditory pathway. This project screens for novel modulators of Cav1.3 (Cui et
al.), which may fundamentally influence the function and localization of these channels in
IHCs.
Photoreceptor Ca2+ channels
In the retina, Cav1.4 Ca2+ channels are required for the tonic release of glutamate from photoreceptor nerve terminals which initiates transmission at the first synapse in the visual pathway. We are identifying mechanisms required for the normal trafficking, localization, and function of Cav1.4 in photoreceptors (make link to Haeseleer et al. PDF, Lee at al. PDF). We are also testing if human mutations in the pore-forming Cav1.4 α1 subunit and a regulatory protein (CaBP4), which cause congenital stationary night blindness 2 (CSNB2), prevent normal trafficking/function of Cav1.4 in photoreceptors.